P. aeruginosa is an important opportunistic human pathogen, meaning that it typically does not infect healthy persons but often infects those already sick or immunocompromised, including those in the hospital.
Our research on this bacterium can divided into three broad areas: biofilm formation, metabolism, and pyocin production.
Biofilm Formation
Biofilms are communities of bacterial cells that are encased in a self-produced extracellular matrix—sort of like a bunch of cells stuck in a gluey substance. Biofilms are important, both in medicine and in industry. Nuisance biofilms can form in pipes and in industrial equipment, while infectious biofilms are difficult for doctors to treat. Because they are encased in a matrix, biofilm cells are relatively shielded from antibiotics, the immune system, and other therapeutics; moreover, because some cells in the interior of a biofilm are not growing, they are especially resistant to antibiotics, making biofilm infections notoriously difficult to eradicate. Therefore, scientists are actively looking for other ways to combat biofilm infections. One of the ways we can do this is to gain a better understanding of how biofilms form—the signals and mechanisms that tell cells to settle down and secrete an extracellular matrix.
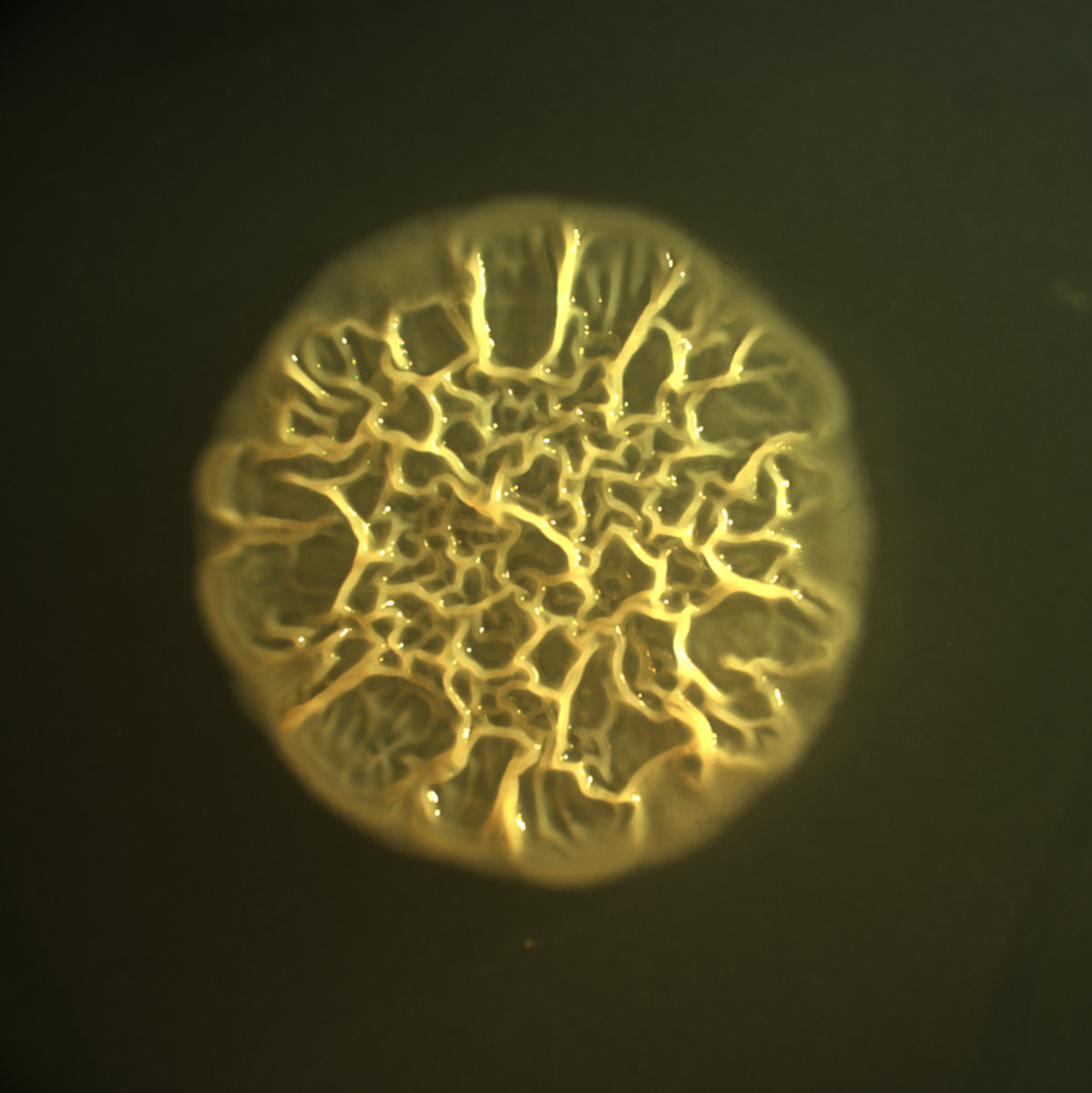
One of the initial research thrusts in the Cabeen lab was to study biofilm formation by P. aeruginosa, which can infect many body sites but is particularly well-known for lung infections, particularly in individuals with cystic fibrosis (a genetic disorder). Because of its medical importance, much is known about how biofilms are signaled and constructed in P. aeruginosa. Upstream signals induce the formation of a bacterial second-messenger compound known as cyclic-di-GMP (cdG), a cyclic dinucleotide molecule that can be thought of as a sort of "internal office memo" in the bacterial cell. High levels of cdG in the cell generally encourage biofilm formation, yet we still have much to learn about all the proteins that control cdG levels or respond to cdG to govern biofilm formation.
We used a screening approach that differs from previous screens in P. aeruginosa. We use colony morphology as a way to assess biofilm formation: wrinkled colonies indicate biofilm formation, whereas smooth and flat colonies indicate that no biofilm has formed (Figure 2). In this way, we can easily assess by visual inspection whether a mutant strain has increased (more wrinkled) or decreased (less wrinkled) biofilm relative to a parental (non-mutant) strain. This approach has uncovered several genes with previously unappreciated roles in biofilm formation, and we continued running the screen to uncover even more genes with roles in biofilm formation while following up on the genes we have already discovered.
This approach has been fruitful, and we currently have manuscripts in preparation or revision in which we characterize genes with previously unappreciated roles in biofilm formation.
Metabolism
Postdoctoral fellow (2020-2022) Simon Underhill has been leading a research direction in the lab exploring signaling and metabolic pathways in P. aeruginosa. He first examined the utilization of citrate and cis-aconitate by P. aeruginosa, finding a number of previously unknown citrate-utilization proteins that are most likely membrane-bound transporters and uncovering extensive redundancy in citrate transport. Teaming up with PhD student Somalisa Pan, he also examined phosphorylation in the nitrogen-related phosphotransfer system (Nitro-PTS) of P. aeruginosa, for the first time showing native phosphostates of the PtsN protein and uncovering new putative regulatory targets of PtsN (Underhill, Pan et al., in revision). Finally, before leaving the lab he conducted experiments linking growth on glycerol with biofilm formation and worked out some of the glycerol metabolism pathway (Underhill et al., in preparation).
Pyocin Production
Pyocins are interbacterial but intraspecific killing molecules. The best-studied among them are the R-type pyocins, which are like phage tails without heads that literally spear competing cells with a microscopic, iron atom-tipped spike, depolarizing the membrane and killing them. While studying a biofilm-related gene, we unexpectedly discovered that P. aeruginosa strains deficient in or deleted for a tyrosine recombinase called XerC overproduce pyocins, which are typically only produced in response to DNA damage via the RecA protein. This second, non-canonical, RecA-independent pathway for pyocin expression is now a major research focus in our lab, as we seek to learn the molecular mechanism of non-canonical pyocin expression and to leverage it for therapeutic advantage.
